Biotracker ™ for NGs..
Next Generation Sequencing (NGS) technologies include a number of methods that are grouped broadly as sample/template preparation, Library Construction, Clusters/Amplification, Sequencing and imaging, and data analysis. The unique combination of specific protocols distinguishes one technology from another and determines the type of data produced from each platform. These differences in NGS methods raised a need for powerful laboratory information system that can track, integrate and share various processes, resources, data, logistics, compliances and standards.
This is where the traditional LIMS systems have predominately failed to meet the NGS lab information management expectations. Unlike other LIMS vendors, Ocimum’s proprietary revolutionary LIMS platform- Biotracker™ LIMS has been a benchmarking standard for Genomic technologies and equally extended its legacy capabilities to better fit for NGS LIMS requirements that can cater to the present and future needs of emerging NGS technologies.
Why Ocimum’s NGS LIMS
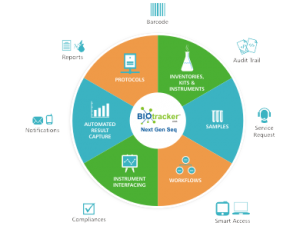
Ocimum Biotracker™ LIMS is one of the best “All in one NGS LIMS” that has been tailored to address the following NGS lab informatics needs
- Lower time, cost and infrastructure required when you choose to use Preconfigured Genomics workflows.
- All in one Genomic LIMS supports Affymetrix, Illumina, Life Tech/ABI’s, Agilent, and other workflows of Genotyping, Gene Expression and Sequencing.
- Powerful configuration tools for quick and efficient modification needs in preconfigured workflows or building a new workflow.
- Better collaboration with Lab staff, Customers, Collaborators and Vendors while providing a role based access to all stake holders.
- Easy and Efficient Genomic Lab specific, Samples, Inventory, Kits, Instruments, Resource and Data management.
- Uni and Bi-directional Instrument interfacing with Sample QC, Hybridization, Plate and Chip transfers, and Scanner machines for faster and secured data capturing.
- Interfacing and automation capabilities through APIs with 3rd party systems within and outside the Genomics facility.
- Scalable and Pluggable with other Genomic workflows and Technologies such as NGS and Biorepositories.
- Retrieve and Report through powerful Reporting engine and search capabilities.
- Guaranteed User experience with fully Web based and easy to use UI for scientific and technical resources.
- Custom build to fit for R&D, Academic cores, Pharma, CRO and Personal Genomics facilities.
TECHNOLOGIES AND PRE-CONFIGURED WORKFLOWS
Vendor |
Instrument |
Inventory, Protocols and Workflows supported |
|
Illumina |
GA11 , GA11X |
Inventory and Equipment: Flow cells, Inventory, Kits, Instruments, |
|
HiSeq 2000, 2000 v3 |
||
|
MiSeq |
||
|
Roche/454 |
GS FLX Titanium |
Inventory: Inventory, Kits, Pico Titer Plates |
|
Life Technologies |
SOLiD 4 |
Protocols: Library prep, Emulsion PCR, Flow chip prep(6 lane), Ligastion Sequencing, Primer Reset |
|
Ion Torrent |
Protocols: Sample Prep, Select Targets, Construct Libraries, Prepare Template, Sequencing, Analyze Data |
|
|
AB1 3700, 3730XL, 3500, 3130 |
Inventory: 96,384plates, Plate transfers, Cherry picking, Inventory, Kits, |
|
| Pacific Bio | RS System | Protocols: Sample Prep, Library/Polymerase complex prep (SMART cell), Synthesis Sequencing Post Sequencing: Movie1>>Raw reads, Post-filter reads, Mapped reads, Movie2>>Raw reads, Post-filter reads, Mapped reads Applications: De Novo Assembly, Targeted Sequencing, Base Modification Detection. |
KEY FEATURES AND MODULES
Genomics specific modules
- Genomic Lab Inventory, Inventory restock, Kits management and usage tracking
- Plate preparation, Transfers (transfer rules, cherry picking), Plate normalization
- Sample pooling, multiplexing, primers/probes, Assay validation, plates/arrays/chips preparation
- Preconfigured Genomic workflows – Sample prep, Sample QC, Plate prep, PCR/Amplification, Cleanup, Sequencing/Genotyping/Expression plate prep, Scanning, Results capture and Results
- Uni and Bi-directional interfacing Affymetrix, Illumina, Life Tech/ABI’s, Agilent, and other instruments
- Post sequencing automatic data capture of QC runs, Reads, Sequence files, .abi, .fsa and more
- NGS reports- Sample flow, cluster reports, sequencing reports, QC reports, service request, Costing and Invoice reports
Compliance
- Audit trails, User logs, System logs, GLP and GXP, 21, CFR Part 11, CLIA*, MIAME, AGCC/GCOS
Functional Modules
- ample Management – full sample genealogy, sample custody, sample pooling, Identification, Aliquot, pooling, derive
- Instrument Management – Vendor details, procurement details, qualification and maintenance schedules and alerts, up & down time tracking
- Scheduling – Instruments, Resources, Samples and Test
- Customer/PI Service request Management
- Customer and Technology based Invoice
- Pre configured, automated reports
Configuration
- SOP/Protocol Management
- Workflow
- Module Design
- Instrument Interfacing
- Third party software interfacing
- Report and Invoice templates
Administration
- Organization, Facility users, groups management
- Role Management- predefined roles, configurable roles for lab staff, customer, collaborators
- Customer, PI and PI staff, Vendor and Collaborators Management
- Utilities – ID & 2D Barcodes, Tasks list, Dashboards, Email and SMS notifications
- Administrator – Manage settings, configurations, fields, modules, vocabulary
Software Functions
- Web based, browser-independent solution
- Works on tablet PCs and Smart phones
- Offered as “On premise” solution and “SaaS” model
- API’s for easy, faster and secured data exchange with third party software and instruments
- Database Independent
