Biotracker™ for Biobanking
Biobanking/Biorepositories is one of the rapidly growing verticals in life science pipeline aiding translational research and pre-clinical trials. The growing global regulatory compliance and long term data traceability coupled with the complexity of sample handling are posing serious problems to both management and technicians.
Ocimum with its vast experience in serving to top-notch customers around the globe has tailored “Ready to use Biobanking LIMS” product to streamline and manage information in regulatory complaint Biobanking and non regulatory Biorepositories. System is sample centric, and capable of tracking information throughout the sample lifecycle.
Biotracker™ Biobanking LIMS is specifically addresses the needs of accurate data capture, secure data storing, effective location and Biospecimen handling management, real-time reporting of data, and maximum operational efficiency. It enables dependable transmission of information between different Biobanks and various clinical and health registries. Productivity, throughput, and accuracy increase, all while improving data administration, sample traceability, and regulatory compliance.
The Biotracker™ for Biobanking solution allows your organization to “collect, store, process and distribute” biological materials in most efficient, secured, compliant and cost effective manner.
Why Ocimum’s Biobanking LIMS
- Fully web based, ready to use, compliant and non-compliant LIMS in the market
- One LIMS product for both Biobanking and Biorepositories need
- Predefined workflows to handle both core Biobanking facility and Sample service request
- Successfully delivered and in use at World’s largest Pharmaceuticals, CRO’s and Medical research centers.
- Highly configurable modules, forms, attributes, privileges and functions are in full control of Administrative user
- API’s for easy, fast and secured data exchange with Freezers, Logistics, Vocabulary and other Software and Database systems
- Experienced and qualified functional, technical and business team to handle the customization needs
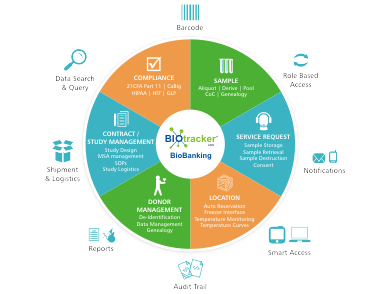
KEY FEATURES AND MODULES
Biobanking Specific Modules
- Contract/Study Management
- Consent
- Sample Shipment and Logistics
- Sample storage, retrieval and destroy service requests
- Sample meta data storage
- Sample import and Export
- Aliquot, Derive and Pool
- Sample Genealogy
- Sample Retrieval and Restocking
- Barcodes
- Sample stocking and destocking sheets
- Automatic Freezer and Location reservations
- Compliant based location storage
- Freezers Interfacing
- Temperature Curves
- Location Management
- Organizational and Global vocabulary
- Role based access to modules and Samples
Administration Modules
- Utilities – ID & 2D Barcodes, Tasks list, Dashboards, Email and SMS notifications
- Administrator – Manage settings, configurations, fields, modules, vocabulary
Configuration
- Donor, Sample and Derivatives modules design
- Modules, Forms, Fields and Page layouts design
- Contract and Study design
- Notification and audit design
- Genealogy design
- Scheduler
- Notifications and Alerts
Compliance
- Audit trails, User logs, GLP and GXP, 21 CFR Part 11,
- Chain of Custody (CoC)
- HIPPA, HL7, SNOMED
Software Functions
- Web based, browser-independent solution
- Works on tablet PCs and Smart phones
- Offered as “On premise” solution and “SaaS” model
- APIs for easy, faster and secured data exchange with third party software and instruments
- Database Independent
FACILITIES AND WORKFLOWS SUPPORTED
- Translational research centers in Pharmaceuticals
- Private Biobanks and Biorepositories
- Medical and Clinical Research
- CRO’s
- Academic research
- Government Sample banks
- Diagnostics Centers
- Forensic
