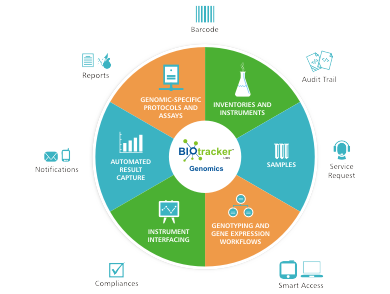
Biotracker™ One LIMS for all Genomic Processes
A Genomic lab processes multiple samples or projects based on contracts and requests, these experiments further are run on various Genotyping, Expression and Sequencing technologies. The resultant data is large, complex, and has various data standards and formats making the data and process management challenging. The data must be captured, securely stored, analyzed, retrieved and shared efficiently across all stake holders within and outside the Facility.
Whether it is an Academic core facility, Big Pharma R&D, Hospital, CRO or Government R&D facility, the Genomics data and process management have raised the potential opportunities to automate several steps of the Genomic process leading to increased throughput.
Ocimum’s Biotracker Genomic LIMS is one such early adopted off the shelf LIMS, that efficiently handles the large amounts of Genomic data generated and helps with quality control, tracking of samples, capture and management of data at different steps of the processes, while serving to manage the workflows precisely. Biotracker also supports good laboratory practices by standardizing protocols, recording and annotating data from every step of the workflow.
Why Ocimum’s GENOMICS LIMS
- Lower time, cost and infrastructure required when you choose to use Preconfigured Genomics workflows.
- All in one Genomic LIMS supports Affymetrix, Illumina, Life Tech/ABI’s, Agilent, and other workflows of Genotyping, Gene Expression and Sequencing.
- Powerful configuration tools for quick and efficient modification needs in preconfigured workflows or building a new workflow.
- Better collaboration with Lab staff, Customers, Collaborators and Vendors while providing a role based access to all stake holders.
- Easy and Efficient Genomic Lab specific, Samples, Inventory, Kits, Instruments, Resource and Data management.
- Uni and Bi-directional Instrument interfacing with Sample QC, Hybridization, Plate and Chip transfers, and Scanner machines for faster and secured data capturing.
- Interfacing and automation capabilities through APIs with 3rd party systems within and outside the Genomics facility.
- Scalable and Pluggable with other Genomic workflows and Technologies such as NGS and Biorepositories.
- Retrieve and Report through powerful Reporting engine and search capabilities.
- Guaranteed User experience with fully Web based and easy to use UI for scientific and technical resources.
- Custom build to fit for R&D, Academic cores, Pharma, CRO and Personal Genomics facilities.
TECHNOLOGIES AND PRE-CONFIGURED WORKFLOWS
| Vendor | Technology and Instruments |
|
Life Technologies, ABI |
Taqman 7900 HT, ABI 3730xl, 3730, 3500xl,3500 |
|
Affymetrix |
Affymetrix GeneChip, GeneChip® Command Console® Software (AGCC)compatible |
|
Illumina |
BeadXpress, BeadArray (HiScan and iScan) |
| Sequenom | MassARRAY Nanodispenser, MassARRAY Analyzer |
| Thermo Scientific | Nanodrop -1000, 2000 and 8000 |
| Agilent | Agilent Gene Spring, Bioanalyzer 2100, 2200 |
| Beckman Coulter | SNPstream®, BioMek FX & NX |
| Qiagen | AutopureLS |
| Hitachi | Spectrophotometer U-3010/3310 |
| LI-COR | LI-COR |
KEY FEATURES AND MODULES
Genomics Specific Modules
- Genomic Lab Inventory, Inventory restock, Kits management and usage tracking
- late preparation, Transfers (transfer rules, cherry picking), Plate normalization
- Sample pooling, multiplexing, primers/probes, Assay validation, plates/arrays/chips preparation
- Preconfigured Genomic workflows – Sample prep, Sample QC, Plate prep, PCR/Amplification, Cleanup, Sequencing/Genotyping/Expression plate prep, Scanning, Results capture and Results
- Uni and Bi-directional interfacing Affymetrix, Illumina, Life Tech/ABI’s, Agilent, and other instruments
- Post sequencing automatic data capture of QC runs, Reads, Sequence files, .abi, .fsa and more
- NGS reports- Sample flow, cluster reports, sequencing reports, QC reports, service request, Costing and Invoice reports
Compliance
- Audit trails, User logs, System logs, GLP and GXP, 21, CFR Part 11, CLIA*, MIAME, AGCC/GCOS
- Sample Management – full sample genealogy, sample custody, sample pooling, Identification, Aliquot, pooling, derive
- Instrument Management – Vendor details, procurement details, qualification and maintenance schedules and alerts, up & down time tracking
- Scheduling – Instruments, Resources, Samples and Test
- Customer/PI Service request Management
- Customer and Technology based Invoice
- Pre configured, automated reports
Functional Modules
Configuration
- SOP/Protocol Management
- Workflow
- Module Design
- Instrument Interfacing
- Third party software interfacing
- Report and Invoice templates
Administration
- Organization, Facility users, groups management
- Role Management- predefined roles, configurable roles for lab staff, customer, collaborators
- Customer, PI and PI staff, Vendor and Collaborators Management
- Utilities – ID & 2D Barcodes, Tasks list, Dashboards, Email and SMS notifications
- Administrator – Manage settings, configurations, fields, modules, vocabulary
Software Functions
- Web based, browser-independent solution
- Works on tablet PCs and Smart phones
- Offered as “On premise” solution and “SaaS” model
- API’s for easy, faster and secured data exchange with third party software and instruments
- Database Independent
Various pricing models are available for Academic, Research, Industry, Hospital, Government and Other organizations. Please contact: lims@ocimumbio.com
